The formats of DNA-encoded libraries (DELs) based on the number and/or assembly of pharmacophores are single-pharmacophore, dual-pharmacophore DELs, and the recent trio-pharmacophore DELs. Trio-pharmacophore DELs, also named triplex pharmacophore DELs, have attracted attention based on encoded self-assembly strategies, and its design is shown in Fig. 1 [1,2]. Compared with dual-pharmacophore DELs, Trio-pharmacophore DELs are a larger self-assembled DELs, and each library member can be purified and characterized [3].
Alfa Chemistry has established the DELs technology platform, enabling us to provide construction services for DELs in different formats. We are investigating and exploring trio-pharmacophore DELs construction strategies in addition to single- and dual-pharmacophore DELs. If you need trio-pharmacophore DELs construction services, please feel free to contact us. With our ongoing exploration of science's frontiers, our research team feels confident in providing you with the services you need.
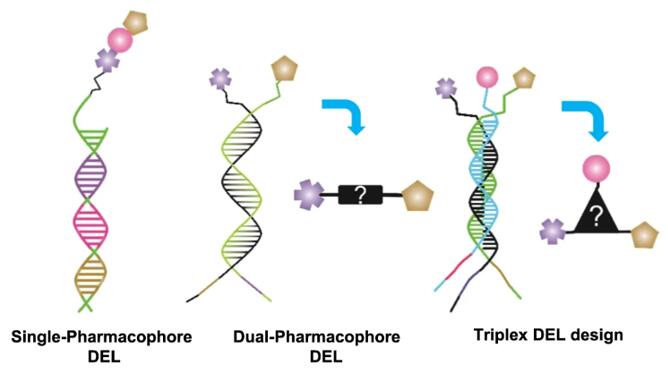 Fig. 1 Schematic representation of a single-pharmacophore, a dual-pharmacophore, and a triplexDELs [3]
Fig. 1 Schematic representation of a single-pharmacophore, a dual-pharmacophore, and a triplexDELs [3]
Difficulties in Constructing Trio-Pharmacophore DELs
Different formats of encoded self-assembly chemistry (ESAC) libraries have been used and suggested for the construction of very large libraries, such as triplex libraries of oligonucleotide conjugates, that is trio-pharmacophore DELs. Triplexes of oligonucleotides could remain stable due to base stacking and Hoogstein hydrogen-bonding. However, the trio-pharmacophore DELs presents some difficulties or challenges, as listed below, that impede its construction [2]:
- It is difficult to find the optimal linkage between two chemical fragments, making the construction of a trio-pharmacophore DELs, which requires the assembly of three chemical fragments, even more challenging.
- For the trio-pharmacophore DELs, site-specific synthesis of multivalent molecules with suitable organic scaffolds is challenging.
Our Services
As part of our construction services, Alfa Chemistry can construct trio-pharmacophore DELs of m × l × n members for our customers by assembling three prepurified sublibrary (SL) libraries: SL-A library with m members, SL-B library with l members, and SL-C library with n members. Fig. 2 shows an assembly diagram of the trio-pharmacophore DELs. Three key points in the construction of a trio-pharmacophore DELs are outlined below.
- It is worth noting that there should be partially complementary base pairs between the sub-library SL-A and SL-C, so that they can form dynamic dual-pharmacophore DELs.
- Besides, SL-B that serves as a scaffold can be used to assemble these two sub-libraries, which can not only mediate distances between fragments in SL-A and SL-C, but also introduce additional contacts with protein targets [1,3].
- What's more, the members in sub-libraries SL-A and SL-C should also have certain base pairs complementary regions with the members in SL-B, and the trio-pharmacophore DELs can be assembled by mixing and annealing the three sublibraries.
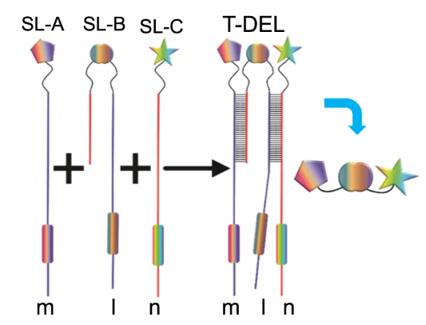 Fig. 2 An assembly diagram of the trio-pharmacophore DELs (T-DELs) [3].
Fig. 2 An assembly diagram of the trio-pharmacophore DELs (T-DELs) [3].
The synthesis of SL-B
The crucial step in the construction of a trio-pharmacophore DELs lies in the synthesis of SL-B, and the process is as follows [3].
a) Conjugate building blocks with oligonucleotides with functional groups at the 3' and 5' ends, respectively, to form a single-side compound-DNA conjugate.
b) Prepare DNA-compound-DNA conjugates containing both oligonucleotides through a DNA-templated reaction, which combines two unilateral DNA conjugates.
c) Encode each conjugate by using adapter DNA and T4 DNA ligase.
d) Purify the encoded conjugates which are library members from the ligation solution.
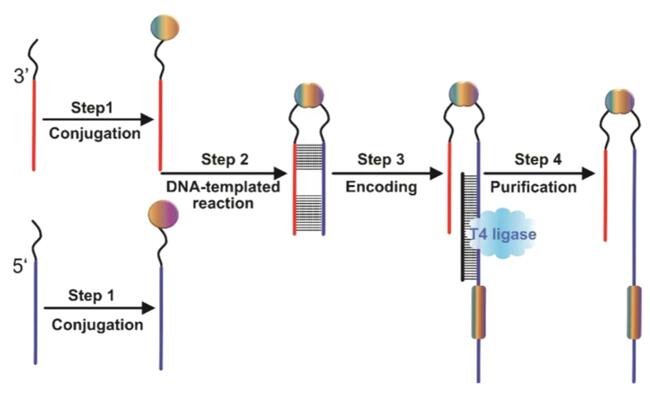 Fig. 3 Synthetic scheme of SL-B [3].
Fig. 3 Synthetic scheme of SL-B [3].
Alfa Chemistry is committed to screening promising compounds through DELs technology to facilitate the discovery of new drugs. Our rich experience in DNA-compatible chemical reaction development and library design strongly supports us in constructing several formats of DELs, including single-pharmacophore DELs, dual-pharmacophore DELs and trio-pharmacophore DELs. Please contact us and we are happy to assist you with any needs you may have.
References
- Melkko, S.; et al. Encoded self-assembling chemical libraries. Nat. Biotechnol. 2004, 22: 568–574.
- Melkko, S.; et al. Lead discovery by DNA-encoded chemical libraries. Drug Discov. Today. 2007, 12: 465–471.
- Cui, M.; et al. Trio-pharmacophore DNA-encoded chemical library for simultaneous selection of fragments and linkers. Nature Communications. 2023, 14: 1481.
