Dual-pharmacophore DNA-encoded libraries (DELs) is a variant of DELs, and the schematic diagram is shown in Fig. 1. In contrast to single-pharmacophore DELs, in which only one chemical moiety is linked to the DNA, in dual-pharmacophore DELs, pairs of compounds/chemical moieties are covalently linked to a single DNA strand. It consists of two sets of partially complementary oligonucleotides, each containing a unique DNA barcode, and two sets of oligonucleotides are mixed together to form a heteroduplex. Due to the close proximity of two independent chemical building blocks, dual pharmacophore DELs can be used to explore adjacent epitopes on a selected drug target, thereby discovering pairs of fragments that bind simultaneously via a chelation effect [1,2].
Alfa Chemistry has a complete DELs technology platform, and our experienced researchers can provide construction services for dual-pharmacophore DELs, such as ESAC libraries and dynamic analogues of the ESAC-type libraries, by self-assembly of partially complementary DNA strands and dynamic recombination, respectively.
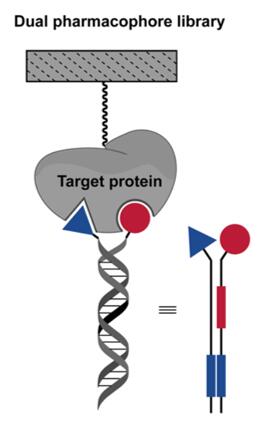 Fig. 1 Format of dual-pharmcophore DELs [2].
Fig. 1 Format of dual-pharmcophore DELs [2].
Our Services
Dual-pharmacophore DELs is also known as encoded self-assembling combinatorial (ESAC) library, which are usually constructed on the basis of synthetic strategies, mainly represented by ESAC libraries. We are capable of constructing dual-pharmacophore DELs, such as ESAC libraries and dynamic analogues of the ESAC-type libraries, which are listed below.
Construct Services of ESAC Libraries
| Approach: Self-assembly of partially complementary DNA strands |
Dual pharmacophore libraries are assembled by hybridization of individually synthesized sublibraries A and B (in case of a two building block library). The first sub-library displays chemicals at the 5' extremity of a tagged oligonucleotide, whereas the second sub-library bears chemical moieties attached at the 3' extremity and features abasic portions to facilitate the hybridization of complementary strands. They are self-assembled by DNA hybridization to form stable individual DNA heteroduplexes, which display pairs of building blocks. Upon annealing between the code A and the d-spacer oligonucleotide, the code B is transferred onto the complementary strand by Klenow polymerization. Since individual oligonucleotide conjugates of sub-libraries are HPLC-purified, MS-analyzed and mixed in equimolar amounts, contributing to an extremely high degree of purity to the resulting assembled dual-pharmacophore DELs [3,4]. |
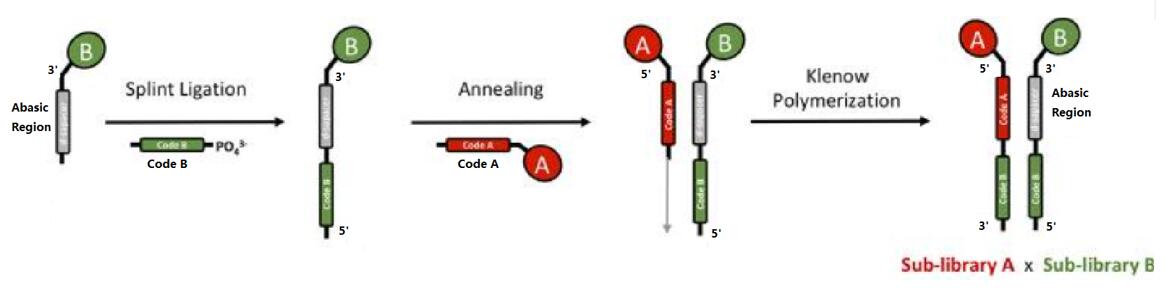 Fig. 2 Construction of ESAC libraries by self-assembly of partially complementary DNA strands [3].
Fig. 2 Construction of ESAC libraries by self-assembly of partially complementary DNA strands [3].
Construct Services of Dynamic Analogues of the ESAC-Type Libraries
| Approach: Dynamic recombination |
Dual pharmacophore liDynamic combinatorial chemistry (DCC) is a method to construct dynamic systems of transient small-molecule adducts under thermodynamic control. With two sublibraries have relatively short complementary DNA strands, a heat-induced DNA-encoded dynamic combinatorial chemical library (hi-EDCCL) can be constructed [5,6]. Due to thermodynamic instability, the unstable double-stranded DNA duplex formed by hybridizing of two sublibraries, and the addition of the target displaces the equilibrium toward the high-affinity combinations. After a round of selection with an immobilized target, the non-binding library pairs can be shuffled by heating and melting the DNA pairs, resulting in new sets of library members that can undergo a second round of selection [3-5]. |
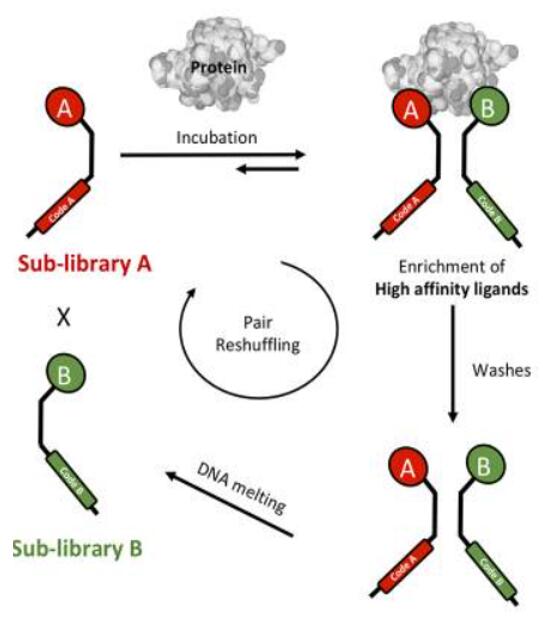 Fig. 2 Construction of hi-EDCCLs which are based on partial annealing of two complementary sub-libraries [3].
Fig. 2 Construction of hi-EDCCLs which are based on partial annealing of two complementary sub-libraries [3].
- The following strategies were developed for hi-EDCCLs:
a) Photo-crosslinking can be applied to lock the equilibrium of DNA-encoded dynamic chemical libraries [6].
b) Y-shaped DNA construct can be designed for the dynamic enrichment of synergistic binding pairs [7].
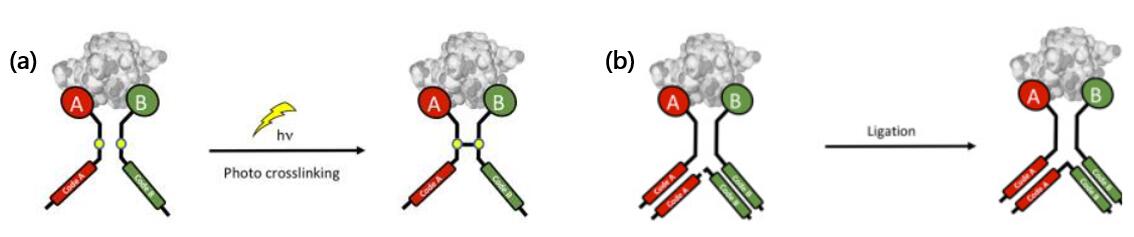 Fig. 3 Schematic representation of different hi-EDCCL approaches [3].
Fig. 3 Schematic representation of different hi-EDCCL approaches [3].
Alfa Chemistry is committed to screening promising compounds through DELs technology to facilitate the discovery of new drugs. Our rich experience in DNA-compatible chemical reaction development and library design strongly supports us in building several formats of DELs, including single-pharmacophore DELs, dual-pharmacophore DELs and trio-pharmacophore DELs. Please contact us and we are happy to assist you with any needs you may have.
References
- Scheuermann, J.; Neri, D. Dual-pharmacophore DNA-encoded chemical libraries. Current Opinion in Chemical Biology. 2015, 26: 99-103.
- Favalli, N.; et al. DNA-encoded chemical libraries: achievements and remaining challenges. FEBS Lett. 2018, 592(12): 2168-2180.
- Adrián, G.-M.; et al. DNA-encoded chemical libraries: A comprehensive review with succesful stories and future challenges. ACS Pharmacol Transl Sci. 2021, 4(4): 1265-1279.
- Madsen, D.; et al. An overview of DNA-encoded libraries: A versatile tool for drug discovery. Prog Med Chem. 2020, 59:181–249.
- Mannocci, L; et al. 20 years of DNA-encoded chemical libraries. Chemical Communications. 2011, 47(48): 12747–12753.
- Zhou, Y.; et al. DNA-Encoded Dynamic Chemical Library and Its Applications in Ligand Discovery. J. Am. Chem. Soc. 2018, 140: 15859–15867.
- Reddavide, F. V.; et al. Second generation DNA-encoded dynamic combinatorial chemical libraries. Chem. Commun. 2019, 55: 3753–3756.
