Single-pharmacophore DNA-encoded libraries (DELs) refers to a type of DELs in which individual organic molecules (no matter how complex) are attached to distinctive DNA fragments, as shown in Fig. 1. In other words, they are the result of the coupling of one DNA fragment to a chemical building block. To date, single-pharmacophore libraries containing thousands to billions of compounds have been synthesized by scientists, and some single-pharmacophore DELs have been used to screen for protein targets [1].
Alfa Chemistry boasts a comprehensive DELs technology platform, and our expert team offers construction services for single-pharmacophore DELs by the combinatorial assembly of chemical building blocks using split-and-pool synthesis (DNA-recorded synthesis) or by the use of complementary oligonucleotide derivatives which facilitate chemical reactions (DNA-templated synthesis). With our ongoing exploration of science's frontiers, our research team feels confident in providing you with the services you need.
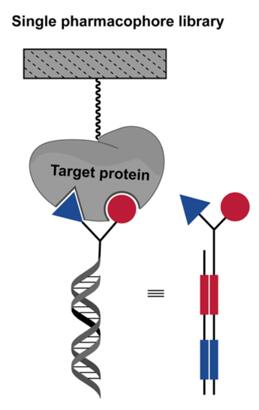 Fig. 1 Format of single-pharmcophore DELs [2].
Fig. 1 Format of single-pharmcophore DELs [2].
Our Strategies
Compared with dual-pharmacophore DELs, single-pharmacophore DELs are simple to design, have a shorter synthesis process, and may yield higher hit rates against specific targets. Numerous strategies have been developed for the construction of single-pharmacophore DELs, which can essentially be classified as DNA-recorded synthesis strategies based on a split-and-pool approach, and DNA-templated synthesis strategies. Alfa Chemistry has the capabilities to offer construction services for a single-pharmacophore DELs by employing these diverse synthetic strategies.

Service Process
The construction process for the different strategies is shown below.
DNA-recorded synthesis
Single-pharmacophore DELs are generally assembled in solution via DNA-recorded synthesis formats based on the combination of split-and-pool procedures and stepwise DNA-tagging, which have the following sequential steps [1,2]:
a) The first set of building blocks is coupled at the end of a corresponding oligonucleotide, featuring a distinctive central sequence as barcode;
b) Compounds produced by the first reaction step are pooled and split into separate reaction vessels, allowing the execution of reactions with a second set of building blocks;
c) DNA-coding for the second reaction step is incorporated by the use of a splint ligation procedure;
d) A three-building block library requires three sets of chemical transformations. Notably, the chemical identity of each reaction can be recorded by annealing with a partially complementary oligonucleotide, followed by a Klenow polymerization step.
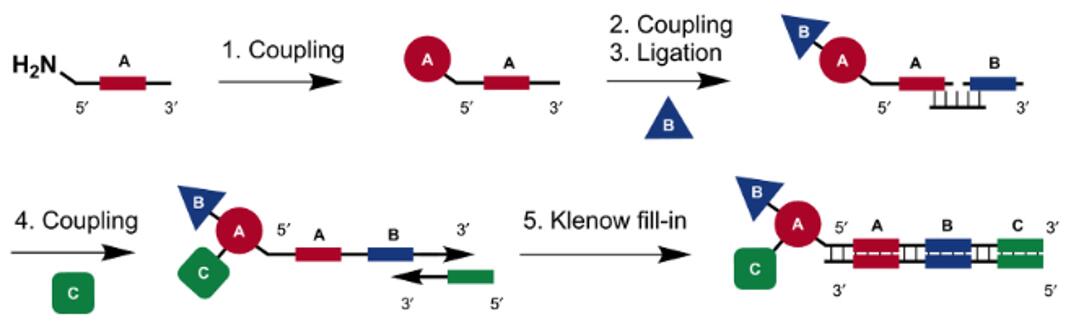 Fig. 2 Schematic representation of strategies for DNA-recorded synthesis (the coding sequences are depicted as A, B, and C) [2].
Fig. 2 Schematic representation of strategies for DNA-recorded synthesis (the coding sequences are depicted as A, B, and C) [2].
DNA-templated synthesis
DNA-templated or DNA-directed synthesis is an alternative strategy for the assembly of single-pharmacophore DELs, which requires the preparation of numerous DNA conjugates, and relies on the use of long presynthesized oligonucleotides, serving as "assembly templates". DNA-templated method accelerates the chemical reaction and controls the formation of chemical products by inducing the two selected reactants to be close together via DNA hybridization, in contrast to the DNA-recorded method. This strategy has been particularly useful for the synthesis of encoded polypeptidic and macrocyclic libraries [1, 2].
- Typical process
In a typical setup, oligonucleotides are used to transfer chemical building blocks to a nascent molecule, attached at the extremity of a complementary DNA strand. After cleavage of the chemical moiety from the reagent oligonucleotide, it can be removed and the template undergoes a new round of template-based synthesis [2].
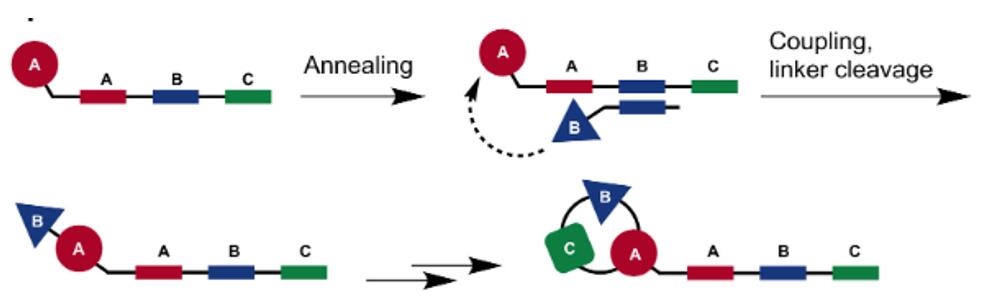 Fig. 3 Schematic diagram of typical DNA-templated synthesis strategies [2].
Fig. 3 Schematic diagram of typical DNA-templated synthesis strategies [2].
- DNA-routing process
DNA-routing is another DNA template-based strategy in which the oligonucleotide mixture is captured on resin containing complementary DNA codons, allowing the selective modification of captured oligonucleotides on the solid phase. Long oligonucleotides are sequentially hybridized to columns carrying complementary oligonucleotides, and individual sequence-specific immobilization reactions decide which building blocks are added to the nascent molecule. In a word, DNA sequences "route" pools of DNA templates into sub-populations [3, 4].
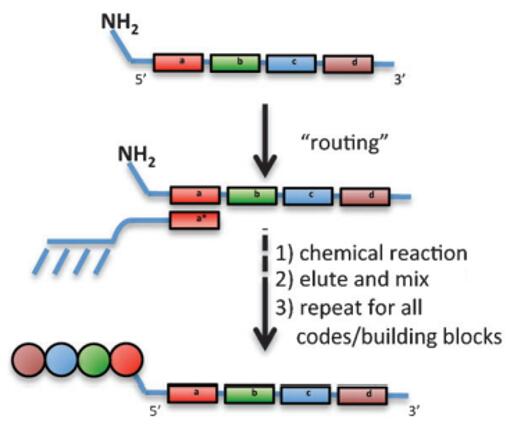 Fig. 4 Schematic diagram of DNA-routing synthesis strategy [3].
Fig. 4 Schematic diagram of DNA-routing synthesis strategy [3].
Alfa Chemistry is committed to screening promising compounds through DELs technology to promote the discovery of new drugs. A variety of strategies can be used to construct single-pharmacophore DELs, including but not limited to those listed on this page, based on our extensive experience in DELs. If you need to construct a single-pharmacophore DELs, please contact us and we will be happy to help you.
References
- Zimmermann, G.; Neri, D. DNA-encoded chemical libraries: foundations and applications in lead discovery. Drug Discov Today. 2016, 21(11): 1828–1834.
- Favalli, N.; et al. DNA-encoded chemical libraries: Achievements and remaining challenges. FEBS Lett. 2018, 592(12): 2168-2180.
- Mannocci, L; et al. 20 years of DNA-encoded chemical libraries. Chemical Communications. 2011, 47(48): 12747–12753.
- Adrián, G.-M.; et al. DNA-Encoded Chemical Libraries: A comprehensive review with succesful stories and future challenges. ACS Pharmacol Transl Sci. 2021, 4(4): 1265–1279.
