 Mlostoń G, et al. Journal of Fluorine Chemistry, 2023, 270, 110170.
Mlostoń G, et al. Journal of Fluorine Chemistry, 2023, 270, 110170.
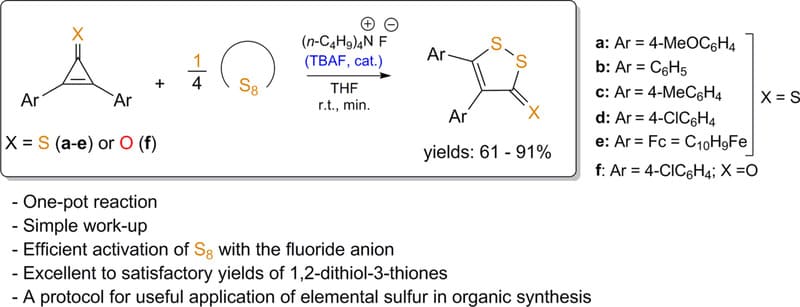
The use of tetrabutylammonium fluoride (TBAF) in the activation of elemental sulfur has proven to be highly effective for the selective sulfurization of various thioketones and ketones. Upon treatment of elemental sulfur with TBAF in THF under an argon atmosphere at room temperature, the sulfur undergoes activation through ring opening, resulting in the formation of a highly reactive fluoropolysulfide anion (FSx‒). This intermediate, characterized by its red color, readily reacts with sterically congested 2,3-diarylcyclopropenethiones, leading to the selective formation of five-membered 3H-1,2-dithiole-3-thiones in excellent yields.
In the experimental procedure, elemental sulfur was suspended in dried THF, and TBAF (1.0 M in THF) was added dropwise. After stirring for 10-15 minutes, the resulting red solution was treated with a thioketone, and the reaction was monitored by TLC until the complete conversion of thione was achieved. The product was purified via silica gel column chromatography, resulting in high yields of the corresponding 3H-1,2-dithiole-3-thiones. Similarly, when 2,3-bis(4-chlorophenyl)cyclopropenone was subjected to the same sulfurization process, the expected 3H-1,2-dithiol-3-one derivative was isolated with an 83% yield.



 Aloiau A. N., et al. The Journal of Organic Chemistry, 2024.
Aloiau A. N., et al. The Journal of Organic Chemistry, 2024.
 Mlostoń G, et al. Journal of Fluorine Chemistry, 2023, 270, 110170.
Mlostoń G, et al. Journal of Fluorine Chemistry, 2023, 270, 110170.
 Wang H, et al. European Polymer Journal, 2020, 140, 109999.
Wang H, et al. European Polymer Journal, 2020, 140, 109999.
 Barik D, et al. Organic Letters, 2025.
Barik D, et al. Organic Letters, 2025.
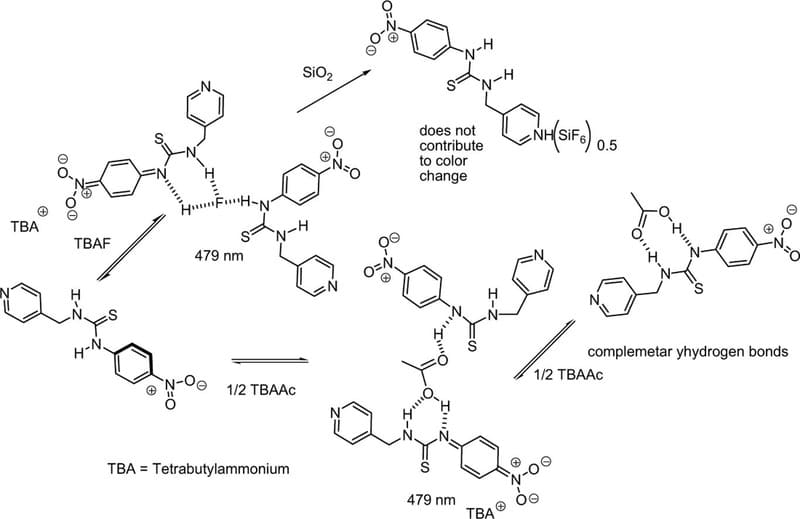 Tarai A, et al. Inorganica Chimica Acta, 2017, 464, 108-113.
Tarai A, et al. Inorganica Chimica Acta, 2017, 464, 108-113.